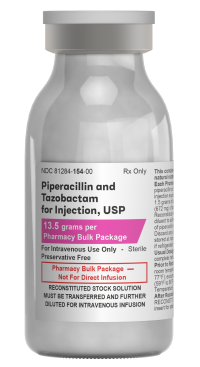
Piperacillin and Tazobactam for Injection, USP
Products > Piperacillin and Tazobactam for Injection, USP
Prescribing Information : Click here
Generic Name
Piperacillin and Tazobactam for Injection, USP
Reference Listed Drug
ZOSYN®
NDC
81284-154-01
Safety Data Sheet
Pack Size
1 Vial
Strength
23 g/2.5 g
Form
13.5 g
GTIN
00381284154109
Therapeutic Class
Anti-infective
Therapeutic Equivalence Rating
AP
Container Closure is not made with natural rubber latex
Preservative Free
YES
Gluten Free
YES
Closure Size
32 mm
Container Size
200 mL
ITEM CODES
Amerisource Bergen
10278085
Cardinal
5833074
McKesson
2689081
Morris & Dickson
261792
<h3><strong>Piperacillin and Tazobactam Injection for Intravenous Injection</strong></h3>
<p> </p>
<p><strong>INDICATION AND IMPORTANT SAFETY INFORMATION</strong></p>
<p><strong>INDICATIONS AND USAGE</strong></p>
<p>Piperacillin and tazobactam for injection is a combination of piperacillin, a penicillin-class antibacterial and tazobactam, a beta-lactamase inhibitor, indicated for the treatment of:</p>
<ul class="normal">
<li>
<p><strong>Intra-abdominal Infections</strong> – in adults and pediatric patients (2 months of age and older) for the treatment of appendicitis (complicated by rupture or abscess) and peritonitis caused by beta-lactamase producing isolates of Escherchia coli or the following members of the Bacteroides fragilis group: B. fragilis, B. ovatus, B. thetaiotaomicron, or B. vulgatus.</p>
</li>
<li>
<p><strong>Nosocomial Pneumonia</strong> – in adults and pediatric patients (2 months of age and older) for the treatment of nosocomial pneumonia (moderate to severe) caused by beta-lactamase producing isolates of Staphylococcus aureus and by piperacillin/tazobactam susceptible Acinetobacter baumannii, Haemophilus influenzae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.</p>
</li>
<li>
<p><strong>Skin and Skin Structure Infections</strong> – in adults for the treatment of uncomplicated and complicated skin and skin structure infections, including cellulitis, cutaneous abscesses and ischemic/diabetic foot infections caused by beta-lactamase producing isolates of Staphylococcus aureus.</p>
</li>
<li>
<p><strong>Female Pelvic Infections</strong> – in adults for the treatment of postpartum endometritis or pelvic inflammatory disease caused by beta-lactamase producing isolates of Escherichia coli.</p>
</li>
<li>
<p><strong>Community-acquired Pneumonia</strong> – in adults for the treatment of community-acquired pneumonia (moderate severity only) caused by beta-lactamase producing isolates of Haemophilus influenzae.</p>
</li>
</ul>
<p>To reduce the development of drug-resistant bacteria and maintain the effectiveness of piperacillin and tazobactam for injection and other antibacterial drugs, piperacillin and tazobactam for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.</p>
<p> </p>
<p><strong>CONTRAINDICATIONS</strong></p>
<p>Piperacillin and tazobactam for injection is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or beta-lactamase inhibitors.</p>
<p> </p>
<p><strong>WARNINGS AND PRECAUTIONS</strong></p>
<p> </p>
<p><strong>Hypersensitivity Adverse Reactions:</strong> Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with piperacillin and tazobactam for injection. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of multiple allergens. Before initiating therapy with piperacillin and tazobactam for injection, careful inquiry should be made concerning previous hypersensitivity reactions. If an allergic reaction occurs, piperacillin and tazobactam for injection should be discontinued.</p>
<p> </p>
<p><strong>Severe Cutaneous Adverse Reactions:</strong> Piperacillin and tazobactam for injection may cause severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis. If patients develop a skin rash they should be monitored closely and piperacillin and tazobactam for injection discontinued if lesions progress.</p>
<p> </p>
<p><strong>Hematologic Adverse Reactions:</strong> Bleeding manifestations have occurred in some patients receiving beta-lactamase drugs, including piperacillin. These reactions are more likely to occur in patients with renal failure. If bleeding manifestations occur, Piperacillin and tazobactam for injection should be discontinued. Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy (21 days or more).</p>
<p> </p>
<p><strong>Central Nervous System Adverse Reactions:</strong> As with other penicillins, piperacillin and tazobactam for injection may cause neuromuscular excitability or seizures. Patients receiving higher doses, especially patients with renal impairment, may be at greater risk for central nervous system adverse reactions. Closely monitor patients with renal impairment or seizure disorders for signs and symptoms of neuromuscular excitability or seizures.</p>
<p> </p>
<p><strong>Nephrotoxicity in Critically Ill Patients:</strong> The use of piperacillin and tazobactam for injection was found to be an independent risk factor for renal failure and was associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs. Alternative treatment options should be considered in the critically ill population. Monitor renal function during treatment of Piperacillin and tazobactam for injection.</p>
<p> </p>
<p><strong>Electrolyte Effects:</strong> Periodic electrolyte determinations should be performed in patients with low potassium reserves, and the possibility of hypokalemia should be kept in mind with patients who have potentially low potassium reserves and who are receiving cytotoxic therapy or diuretics.</p>
<p> </p>
<p><strong>Clostridioides difficile-Associated Diarrhea:</strong> Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including piperacillin and tazobactam for injection, and may range in severity from mild diarrhea to fatal colitis. If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued.</p>
<p> </p>
<p><strong>Development of Drug-Resistant Bacteria:</strong> Prescribing piperacillin and tazobactam for injection in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.</p>
<p> </p>
<p><strong>ADVERSE REACTIONS</strong></p>
<p>The most common adverse reactions (≥ 5%) in clinical trials were: diarrhea, nausea, headache, and insomnia.</p>
<p> </p>
<p><strong>DRUG INTERACTIONS</strong></p>
<p><strong>Aminoglycosides:</strong> Piperacillin may inactivate aminoglycosides by converting them to microbiologically inert amides. Piperacillin and tazobactam for injection is not compatible with tobramycin for simultaneous Y-site infusion.</p>
<p> </p>
<p><strong>Probenecid:</strong> Probenecid prolongs the half-lives of piperacillin and tazobactam and should not be co-administered with piperacillin and tazobactam for injection unless the benefit outweighs the risk.</p>
<p> </p>
<p><strong>Vancomycin:</strong>Co-administration of piperacillin and tazobactam for injection with vancomycin may increase the incidence of acute kidney injury. Monitor kidney function in patients receiving piperacillin and tazobactam for injection and vancomycin.</p>
<p> </p>
<p><strong>Anticoagulants:</strong> Monitor coagulation parameters in patients receiving piperacillin and tazobactam for injection and heparin or oral anticoagulants.</p>
<p> </p>
<p><strong>Vecuronium:</strong> Piperacillin and tazobactam for injection may prolong the neuromuscular blockade of vecuronium and other non-depolarizing neuromuscular blockers. Monitor for adverse reactions related to neuromuscular blockade.</p>
<p> </p>
<p><strong>Methotrexate:</strong> Co-administration of methotrexate and piperacillin may reduce the clearance of methotrexate. If concurrent therapy is necessary, serum concentrations of methotrexate as well as the signs and symptoms of methotrexate toxicity should be frequently monitored.</p>
<p> </p>
<p><strong>Effects on Laboratory Tests:</strong> There have been reports of positive test results using the Bio-Rad Laboratories Platelia Aspergillus EIA test in patients receiving piperacillin/tazobactam injection who were subsequently found to be free of Aspergillus infection. The administration of piperacillin and tazobactam for injection may result in a false-positive reaction for glucose in the urine using a copper-reduction method (CLINITEST).</p>
<p> </p>
<p><strong>IMPORTANT ADMINISTRATION INSTRUCTIONS</strong></p>
<p>Administer piperacillin and tazobactam for injection by intravenous infusion over 30 minutes to both adult and pediatric patients. See Full Prescribing Information for complete dosing and preparation information. Lactated Ringer’s Solution is NOT compatible with piperacillin and tazobactam for injection. Piperacillin and tazobactam for injection should NOT be mixed with other drugs in a syringe or infusion bottle. Piperacillin and tazobactam for injection is not chemically stable in solutions that contain only sodium bicarbonate and solutions that signficantly alter the pH. Piperacillin and tazobactam for injection is not compatible with tobramycin for simultaneous Y-site infusion. Piperacillin and tazobactam for injection should NOT be added to blood products or albumin hydrolysates.</p>
<p> </p>
<p><strong>USE IN SPECIFIC POPULATIONS</strong></p>
<p><strong>Pregnancy</strong> <u>Risk Summary</u>: Piperacillin and tazobactam for injection cross the placenta in humans. However, there are insufficient data with piperacillin and/or tazobactam in pregnant women to inform a<br />
drug-associated risk for major birth defects and miscarriage.</p>
<p> </p>
<p><strong>Lactation</strong> <u>Risk Summary</u>: Piperacillin is excreted in human milk; tazobactam concentrations in human milk have not been studies. No information is available on the effects of piperacillin and tazobactam on the breastfed child or on milk production.</p>
<p> </p>
<p><strong>Pediatric Use</strong>: The safety and effectiveness of piperacillin and tazobactam for injection for intra-abdominal infections, and nosocomial pneumonia have been established in pediatric patients 2 months of age and older. The safety and effectiveness of piperacillin and tazobactam for injection have not been established in pediatric patients less than 2 months of age.</p>
<p> </p>
<p><strong>Geriatric Use</strong>: Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Care should be taken in dose selection, and it may be useful to monitor renal function – in the event of renal impairment refer to the Renal Impairment instructions below and in the full prescribing information.</p>
<p> </p>
<p><strong>Renal Impairment:</strong> This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. In patients with creatinine clearance ≤ 40 mL/min and dialysis patients, the intravenous dose of piperacillin and tazobactam for injection should be reduced to the degree of renal function impairment. See Full Prescribing Information for complete dosing information.</p>
<p> </p>
<p><strong>Hepatic Impairment:</strong> Dosage adjustment of piperacillin and tazobactam for injection is not warranted in patients with hepatic impairment.</p>
<p> </p>
<p><strong>Patients with Cystic Fibrosis:</strong> Piperacillin and tazobactam for injection has been associated with an increased incidence of fever and rash in cystic fibrosis patients.</p>
<p> </p>
<p><strong>OVERDOSAGE</strong></p>
<p>There have been postmarketing reports of overdose with piperacillin/tazobactam. The majority of those events experienced, including nausea, vomiting, and diarrhea, have also been reported with the usual recommended dosages. Patients may experience neuromuscular excitability or seizures if higher than recommended doses are given intravenously (particularly in the presence of renal failure).</p>
<p><strong>For additional Safety Information, please see Full Prescribing Information.</strong></p>
<p><strong>You are encouraged to report adverse drug events to Provepharm at 1-833-727-6556 or to the FDA by visiting www.fda.gov/medwatch or by calling 1-800-FDA-1088.</strong></p>
